Concept
Hypokalemia is defined as the concentration in plasma K + <3 .5="" l.="" li="" mmol="">
- 98% of the potassium (K +) total body is in the intracellular space, mainly in skeletal muscle.
- The amount of K + in the extracellular space is less than 2% of total body potassium
- The ratio of the concentration of K + between intracellular and extracellular fluid is maintained by the resting membrane potential and is essential for normal neuromuscular function.
- About 90% of the K + ingested in the diet is excreted in the urine.
- The factors controlling the distribution of K + are, essentially:
- The pH value causes the output acidosis K + cells and alkalosis otherwise.
- insulin
- aldosterone
- catecholamines
Etiology
Hypokalemia may be due to one or more of the following causes:
- ingestion diminished
- Penetration into cells
- Increased losses final
- ingestion diminished
- Penetration into cells
- Increased losses final
- DIGESTIVE CAUSES LOSS OF POTASSIUM
- Vomiting, gastric aspiration
- diarrhea
- infectious causes
- intestinal tumors
- digestive fistulas
- Zollinger-Ellison Syndrome
- Verner-Morrison syndrome (pancreatic cholera)
- Malabsorption syndrome
- Abuse of laxatives
- Short-circuit jejunal ileus
- Congenital chloride diarrhea
- thiazide diuretics
- other drugs
- antibiotics
- cisplatin
- lithium
- L-dopa
- Thallium Poisoning
- Magnesium depletion
- metabolic alkalosis
- Mineralocorticoid excess
- Primary aldosteronism
- Cushing's syndrome and steroid treatment
- Hiperreninism
- Apparent mineralocorticoid excess
- kidney problems
- Renal tubular acidosis
- Family or idiopathic diseases: Bartter syndrome or Liddle
- other causes
- Diabetic Acidosis
- hypercalcemia
- leukocytosis
- Elevation of extracellular pH
- insulin
- adrenergic drugs
- Familial periodic paralysis
- other causes
- Intoxication by barium and toluene
- Chloroquine poisoning
- Treatment of anemia and neutropenia
- hypothermia
- United Anabolic parenteral nutrition.
- metabolic acidosis
- Acute diarrhea
- ketoacidosis
- Renal tubular acidosis
- chronic pyelonephritis
- metabolic alkalosis
- diuretic therapy
- Vomiting, gastrointestinal suction
- Hipermineralcorticismo
- hypomagnesemia
CLiNIC
- Rarely are no symptoms, except that the concentration of K + in the plasma falls below 3 mmol / L.
- There are situations especially sensitive to hypokalaemia, such as:
- When a rapid depletion of K +
- Taking digoxin
- Previous disease or cardiac neuromuscular
- hypocalcemia
- hypomagnesemia
Neuromuscular Manifestations
- Hypokalemia can produce more intense progressive weakness, hypoventilation (by condition of respiratory muscles) and, finally, complete paralysis.
- The deterioration of muscle metabolism intensifies the risk of rhabdomyolysis, sometimes accompanied by acute renal failure.
- The role of smooth muscle fiber may be affected and manifest ileus.
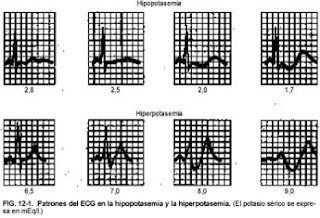
- The earliest changes are flattening or inversion of the T wave, prominent U wave, ST segment depression and prolonged QU interval.
- Severe depletion of K + can produce a prolonged PR interval, decreased voltage and QRS widening, increasing the risk of ventricular arrhythmias (especially in patients with myocardial ischemia).
- Hypokalemia may also predispose to digitalis toxicity
metabolic consequences
Hypokalemia is usually associated with acid-base balance disorders:
- The decrease in K + produces intracellular acidification and increased final disposal of acids or train more HCO3 ¯, which favors the occurrence of metabolic alkalosis.
- It may also appear glucose intolerance, which has been attributed to decreased secretion of peripheral resistance to insulin or the hormone.
- It is not uncommon in nephrogenic diabetes insipidus hypokalemia appears to be manifested by polydipsia and polyuria.
DIAGNOSIS
In most cases, the cause of the decrease in K + can be identified by a suitable anamnesis.
We detect whether there has been abuse of laxatives and diuretics, or if the patient has been vomiting.
Barring poor intake or K + over the intracellular environment as possible causes of hypokalemia, we examine the response of the kidney to clarify the origin of the loss of K +. Adequate renal response to decreased K + urinary clearance is Hypokalemia coupled with minimal removal of K + in the kidney indicates that the K +'re missing:
- For skin
- By the gut
- There will be a history of vomiting or use of diuretics.
But it is clear that this formula is complex to understand and, above all, difficult to implement correctly in the daily job of the emergency physician. The same goes for other determinations as levels of plasma renin and aldosterone in the bicarbonaturia, and the elimination of other non-resorbable anion also increases TTKG and produce renal K + losses.
Therefore, for practical purposes, in the emergency we try to reach a diagnostic approach based on:
- Anamnesis and clinical Sr.: assessing history of vomiting, diarrhea, muscle weakness episodes, taking diuretics or laxatives.
- Physical examination: look
- for signs of volume depletion (vomiting, diuretics), hypertension, etc..
- ECG
- Laboratory Tests:
- CBC
- Biochemistry: glucose, urea, creatinine, Na, K, chloride and calcium
- Biochemistry Urine Na, K
- gasometry
TREATMENT
The therapeutic goals addressed to:
- Correct the K + deficit
- Minimize losses continue to occur
Considerations to take into account:
- In general, it is less "dangerous" correct hypokalemia through oral ingestion of K +.
- The degree of reduction of K + does not keep a close relationship with the [K +] in plasma. Thus, the decrease of 1 mmol / L of K + in the plasma (eg from 4.0 to 3.0 mmol / L) may be a deficit of 200 to 400 mmol of total body K +, and those with less than 3.0 mmol / L of K + in plasma often need more than 600 mmol of K + to correct the deficit.
- Factors favoring the exit of K + from the cells (as in insulin deficiency of diabetic ketoacidosis) can cause an "underestimation" of the K + deficit.
- It is therefore necessary to "monitor" often K + concentration in plasma to assess the response to treatment.
SLIGHT hypokalemia (K + = 3 - 3.5 mmol / L)
Treatment will be aimed at correcting the underlying cause and supplement the diet with foods rich in K + (orange, banana, tomato)
MODERATE hypokalemia (2.5 - 3.0 mmol / L)
Supplements should be provided for K + at a dose of 60-80 mEq / day, administered with food recommended by gastroduodenal ulcer risk.
Pharmacological supplements vary by acid-base status:
- If there is metabolic alkalosis, give potassium ascorbate (Boi-K ®, 1 comp = 10 mEq) or ascorbate-ASPARTAME potassium (K Boi-Aspartic ®, 1 comp = 25 mEq).
- If metabolic acidosis is administered potassium chloride (Potasión ®, 1 comp = 5 mEq) or (Potasión 600 ®, 1 tab = 8 mEq)
Severe hypokalemia (K + <2 b="" nbsp="">5 mmol / L) or intolerance ORAL
Should receive replacement therapy with potassium chloride (CLK amp. 3 ml = 10 mEq) IV diluted in saline (solutions of glucose and bicarbonate redistribute the intracellular space K +).
- The infusion rate should not exceed 20 mmol / h, unless there is paralysis or threatening ventricular arrhythmias life, and in this case must monitor the patient and monitor signs of hyperkalemia and neuromuscular regularly perform an examination.
- The [K +] maximum should not exceed 40 mmol / L when administered through a peripheral vein or 60 mmol / L when using a central, preferably channel a femoral approach, the risk of causing arrhythmias.
- General measures: ECG Monitoring and Monitoring plasma K + every 6 hours
Information obtained in: http://www.dep19.san.gva.es/servicios/urgencias/files/protocolos/hipopotasemia.htm